Answer: -
Molality of NaCl = 2.807 ×10⁻² m
Explanation: -
Molarity given = 2.800×10⁻² M
This means there are 2.800×10⁻² moles of NaCl per 1000 mL of solution.
Volume of water = 999.2 ml
Density of water = 0.9982 g/ml
Mass of water = Density of water x Volume of water
= 0.9982 g/ml x 999.2 ml
= 997.4 g
Thus 997.4 g of water has 2.800×10⁻² moles of NaCl.
1000 g of water has
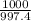 x 2.800×10⁻² moles of NaCl.
x 2.800×10⁻² moles of NaCl.
= 2.807 ×10⁻² moles of NaCl.
Molality of NaCl = 2.807 ×10⁻² m