Atomic number of gold,
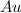 = 79 means there are 79 electrons in per atom of gold.
= 79 means there are 79 electrons in per atom of gold.
Atomic mass of gold = 196.967 gm
0.990 oz of gold is given.
Now, converting
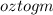

So,
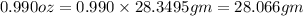
Now, for determining the number of moles, the formula for number of moles is:
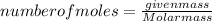
Substituting the values:
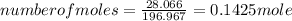
1 mole of
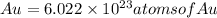
So, for 0.1425 mole of
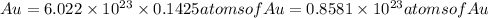
Since 1 atom of gold has 79 electrons so,
Number of electrons =

So, number of electrons in 0.990 oz of pure gold coin is
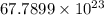 .
.