Answer: -
0.1 ml of bleach should be added to each liter of test solution.
Explanation:-
Let the volume of bleach to be added is B ml.
Density of stock solution = 1.0 g/ml
Mass of stock solution = Volume of stock x density of stock
= B ml x 1.0 g/ml
= B g
Amount of NaOCl in this stock solution = 5% of B g
=
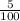 x B g
x B g
= 0.05 B g
Now each test solution must be added 5 mg/l NaOCl.
Thus each liter of test solution must have 5 mg.
Thus 0.05 B g = 5 mg
= 0.005 g
B =
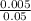
= 0.1
Thus 0.1 ml of bleach should be added to each liter of test solution.