According to law of conservation of mass, the mass can neither be destroyed nor created in a chemical reaction. The mass of reactants and mass of products are equal in a chemical reaction.
The reaction between A and B (reactants) to form C and D (products) is given as:
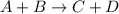
The mass of A =
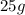 (given)
(given)
The mass of B =
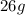 (given)
(given)
The mass of reactant = mass of A + mass of B
Substituting the values:
The mass of reactant =
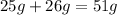
The mass of C =
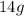
Let the mass of D =
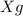
So, the mass of product = mass of C + mass of D
Substituting the values:
the mass of product =
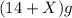
According to law of conservation of mass:
the mass of reactant = the mass of product
Substituting the values,
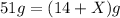
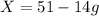
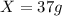
Hence, the amount of D produced in the reaction is
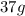 .
.