The reaction for combustion of hydrogen gas in the oxygen is as follows:
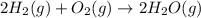
Here, oxygen is present in excess thus, hydrogen will be limiting reactant.
2 moles of hydrogen gives 2 moles of water vapor, thus, 1 mole will give 1 mole of water vapor.
The mass of hydrogen is 1.80 g and molar mass of
 is 2 g/mol converting mass into number of moles:
is 2 g/mol converting mass into number of moles:
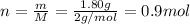
Thus, 0.9 mol of
 gives 0.9 mol of
gives 0.9 mol of
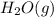 . Molar mass of
. Molar mass of
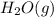 is 18 g/mol, converting number of moles to mass,
is 18 g/mol, converting number of moles to mass,
m=n×M=0.9 mol× 18 g/mol=16.2 g
Therefore, mass of water vapor
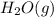 produced will be 16.2 g.
produced will be 16.2 g.