Answer:-
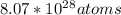
Solution:- It is a unit conversion where it asks to convert kg of He to atoms. We need to convert kg to grams, grams to moles and finally moles to atoms.
We know that, 1 kg = 1000 g
Molar mass of He is 4.0 grams per mole and 1 mole of an atom equals to Avogadro number of atoms.
The set up would be as given below:
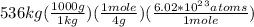
=
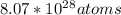
So, there are
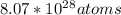 of He in it's 536 kg.
of He in it's 536 kg.