Answer : The correct option is, 68 g
Explanation :
First we have top calculate the mass of solvent (water).
Formula used :
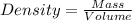
Now put all the values in this formula, we get the mass of solvent (water).
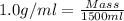
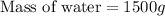
Now we have to calculate the mass of hydrogen peroxide.
Molality : It is defined as the number of moles of solute present in one kilogram of solvent.
In this question, the solute is hydrogen peroxide.
Formula used :
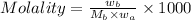
where,
Molality = 1.33 mole/Kg
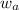 = mass of solvent (water) = 1500 g
= mass of solvent (water) = 1500 g
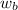 = mass of solute hydrogen peroxide = ?
= mass of solute hydrogen peroxide = ?
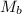 = molar mass of solute hydrogen peroxide = 34 g/mole
= molar mass of solute hydrogen peroxide = 34 g/mole
Now put all the given values in the above formula, we get the mass of hydrogen peroxide.
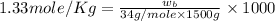
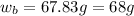
Therefore, the grams of hydrogen peroxide will be, 68 grams.