The formula to calculate will be:
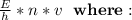
E = energy (11000 J)
H = Planck's constant (6.62607004 × 10-34 m2)
N = number of photons
V = frequency (880)
Solution:
![\frac{11000}{6.62607004 * [tex] 10^(-34)]() } * n * 880 [/tex]
} * n * 880 [/tex]
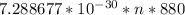
And that is the final step to calculating the answer.
Hope it helped,
BioTeacher101