Step-by-step explanation:
The given data is as follows.
Volume = 3.24 ml, mass = 62.5429 g
As we know that density is the mass of object divided by the volume occupied by it.
Mathematically, Density =
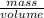
Therefore, calculate the density of given unknown metal as follows.
Density =
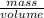
=
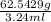
= 19.30 g/ml
Hence, density of the given unknown metal is 19.30 g/ml.
Gold is the element which has a density of 19.30 g/ml.
Thus, we can conclude that given unknown metal has a density of 19.30 g/ml and name of this metal is gold.