The formula of determining the molarity is:
molarity =
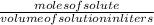 -(1)
-(1)
Volume of solution =
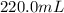 (given)
(given)
Since, 1 mL = 0.001 L
Therefore, volume of the solution = 0.22 L
Molarity = 0.194 M (given)
Substituting the values in formula (1)
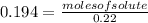
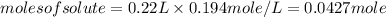
Now, number of moles =
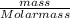 -(2)
-(2)
Molar mass of
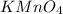 =
=
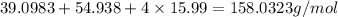
Substituting the value of number of moles and molar mass of
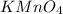 in equation -(2)
in equation -(2)
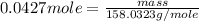
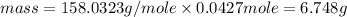
Hence, the mass in grams of potassium permanganate the chemist has added to the flask is
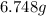 .
.