Answer:- 3.0 g
Solution:- Cyclohexanol gives cyclohexene on acid catalysis. Water molecule is actually given out in the reaction.
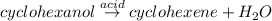
There is 1:1 mole ratio between cyclohexanol and cyclohexene. Let's calculate the theoretical yield of cyclohexene using stoichiometry as:
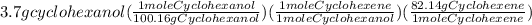
= 3.0 g Cyclohexene
Therefore, the theoretical yield of cyclohexene is 3.0 g.