Density of gasoline is 0.737 g/mL and volume of tank is 13.0 gal.
Since, 1 US gal=3.78 L
Volume of tank in L will be:
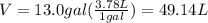
Also, 1 L=1000 mL
Thus,
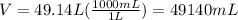
Mass of gasoline can be calculated as follows:
m=d×V
Here, d is density and V is volume thus,
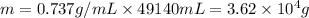
Therefore, mass of gasoline will be
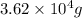 .
.