Step-by-step explanation:
It is known that number of moles of a substance equals mass divided molar mass of the substance.
Mathematically, No. of moles =
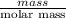
It is given that number of moles of diazepam (valium) is 0.05570 mol and its mass is 15.86 g.
Therefore, molar mass of diazepam (valium) will be calculated as follows.
No. of moles =
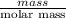
0.05570 mol =
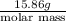
molar mass = 284.74 g
Thus, we can conclude that the molar mass of diazepam (valium) if 0.05570 mol has a mass of 15.86 g is 284.74 g.