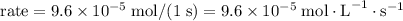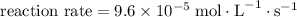
Step-by-step explanation:
Concentration
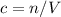 ;
;
The reaction see a
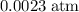 change in pressure per second. By the ideal gas law, this quantity would correspond to a change in concentration,
change in pressure per second. By the ideal gas law, this quantity would correspond to a change in concentration,
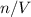 of
of
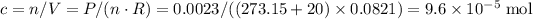
where the ideal gas constant
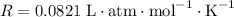 .
.
By definition,
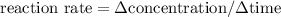
Therefore, for this particular reaction