Answer:-
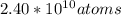
Solution:- It is a simple unit conversion problem. We could solve this using dimensional analysis.
We know that, 1 US dollar = 100 cents
1 cent = 1 US penny
So, 1 US dollar = 100 US pennies
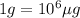
Let's make the set up starting with 1 penny as:
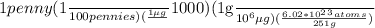
=
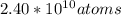
Therefore, we can bye
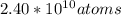 of Cf in one US penny.
of Cf in one US penny.