Step-by-step explanation:
It is known that at
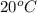 the solubility of sodium chloride per 100 grams of water is 36 grams. Whereas the solubility of lead nitrate per 100 grams of water is 54.3 grams.
the solubility of sodium chloride per 100 grams of water is 36 grams. Whereas the solubility of lead nitrate per 100 grams of water is 54.3 grams.
Therefore, when 200 kg (or 200000 g) of sodium chloride's solubility is 36 then calculate solubility of 100 Kg, that is, 100000 g of sodium chloride as follows.
Solubility =
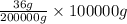
= 18 g
On the other hand, when 100000 g of lead nitrate's solubility is 54 then calculate solubility of 200 Kg, that is, 200000 g of lead nitrate as follows.
Solubility =
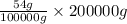
= 108 g
Hence, difference between K and L is as follows.
Difference = solubility of K - solubility of L
= 108 g - 18 g
= 90 g
Thus, we can conclude that K is more soluble than L.