Answer: 1.45 moles of
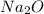
Step-by-step explanation:
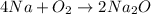
According to the stoichiometry:
Given : Na react completely, thus sodium is a limiting reagent as it limits the formation of product. Oxygen will be excess reagent as it does not react completely.
4 moles of sodium produce 2 moles of
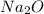
Thus 2.90 moles of sodium produce =
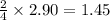 moles of
moles of
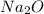
Thus 1.45 moles of
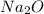 will be produced.
will be produced.