The dissociation reaction of acetic acid is as follows:
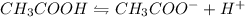
The acid dissociation constant K_{a} is
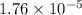 .
.
Let the initial concentration of acid be A, and concentration of
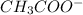 and
and
 be zero.
be zero.
After dissociation, concentration of acid becomes A-x and that of both
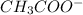 and
and
 becomes x.
becomes x.
Expression for acid dissociation constant will be:
![K_(a)=([CH_(3)COO^(-)][H^(+)])/([CH_(3)COOH])](https://img.qammunity.org/2019/formulas/chemistry/college/a0mo3esfhm8io5sahuk46x8swvjjldi7bd.png)
pH of solution is 3.5, thus, concentration of hydrogen ion can be calculated as follows:
![pH=-log[H^(+)]](https://img.qammunity.org/2019/formulas/chemistry/college/i7qr83payj0m2981ren99sjnsbpwpe4tdh.png)
On rearranging,
![[H^(+)]=10^(-pH)=10^(-3.5)=0.0003162](https://img.qammunity.org/2019/formulas/chemistry/college/huf7ceytzdsl22wc5b9tul1ymniej7kh6u.png)
Since,
![[CH_(3)COO^(-)]=[H^(+)]=x](https://img.qammunity.org/2019/formulas/chemistry/college/k9v9155wztx01z0drtyfe5a24xz1bwqla9.png)
Thus,
![[CH_(3)COO^(-)]=0.0003162](https://img.qammunity.org/2019/formulas/chemistry/college/nj5lpxjj6zw8uczb1edv9lov6tiacnqpzi.png)
and,
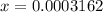
Putting the values, in expression for acid dissociation constant,
![1.76* 10^(-5)=((0.0003162)(0.0003162))/([CH_(3)COOH]_(initial)-0.0003162)](https://img.qammunity.org/2019/formulas/chemistry/college/8bn3ms25egmyjv5w0hlwl9vb1ctepfhya5.png)
On rearranging,
![[CH_(3)COOH]_(initial)=((0.0003162)* (0.0003162))/(1.76* 10^(-5))+0.0003162=0.006](https://img.qammunity.org/2019/formulas/chemistry/college/5ge8pqozv2m23vnzf49mq81nbn41qr2bxe.png)