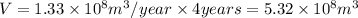The production of
 is
is
 . Converting mass into kg,
. Converting mass into kg,
1 ton=907.185 kg, thus,
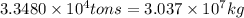
Thus, production of
 will be
will be
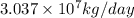 .
.
The specific volume of
 is
is
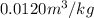 .
.
Volume of
 produced per day can be calculated as:
produced per day can be calculated as:
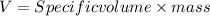
Putting the values,
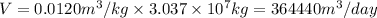
Thus, volume of
 produced per year will be:
produced per year will be:
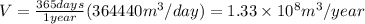
Thus, in 4 year volume of
 produced will be:
produced will be: