Answer :The average atomic mass of Sulfur is 32.066 amu.
Explanation :
The average atomic mass of an element is the total of atomic mass of an isotope multiplied by its natural abundance.
The formula for average atomic mass of S can be written as follows.
Average Atomic Mass = (Atomic mass of S-32 * Abundance of S-32) + (Atomic mass of S-33 * Abundance of S-33) + (Atomic mass of S-34 * Abundance of S-34) + (Atomic mass of S-36 * Abundance of S-36)
For calculation purpose, we will use atomic masses same as mass numbers .
S-32 = 32 amu ; Abundance = 0.95
S-33 = 33 amu ; Abundance = 0.0076
S-34 = 34 amu ; Abundance = 0.0322
S-36 = 36 amu ; Abundance = 0.0089
Let us plug the above values in average atomic mass equation.
Average Atomic Mass =
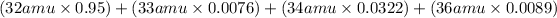
Average Atomic Mass =
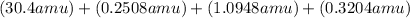
Average Atomic Mass = 32.066 amu
The average atomic mass of Sulfur is 32.066 amu