Answer:- 89.6 mL
Solution:- Mass of empty flask = 123.4 g
mass of flask filled with water = 211.6 g
So, mass of water in the flask = 211.6 g - 123.4 g = 88.2 g
we know that density of water is
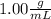 .
.
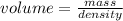
So, volume of water in the flask =
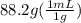
= 88.2 mL
Mass and density is also given for Zn metal. So, we could calculate the volume of metal also using the same formula used for water.
volume of Zn =
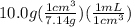
= 1.40 mL
New volume of the flask would be the sum of the volumes of water and metal.
So, new volume of the flask = 88.2 mL + 1.40 mL = 89.6 mL
Hence, the new volume of the flask after adding the metal to it is 89.6 mL.