Here,
Heat gain by the first sample of water + Heat lost by the second sample of water is equal to zero (0).
Now, Mass of water sample one = 108 g (given)
Mass of water sample two = 66.9 g (given)
Temperature for water sample one =
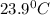
Let, temperature for water sample two =x
And, final temperature =
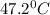
Now,
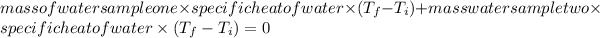
where,
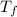 = final temperature
= final temperature
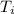 = initial temperature
= initial temperature
Substitute all the given values in above formula:
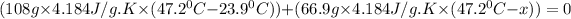
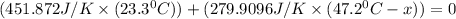
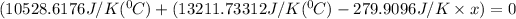
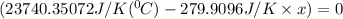
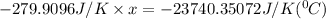
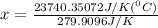
[tex x =84.81^{0}C [/tex]