Answer : The orbitals that are used to form each indicated bond in citric acid is given below as per the attachment.
Answer 1) σ Bond a : C has
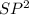 O has
O has
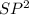 .
.
Explanation : The orbitals of oxygen and carbon which are involved in
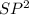 hybridization to form sigma bonds. This is observed at 'a' position in the citric acid molecule.
hybridization to form sigma bonds. This is observed at 'a' position in the citric acid molecule.
Answer 2) π Bond a: C has π orbitals and O also has π orbitals.
Explanation : The pi-bond at the 'a'position has carbon and oxygen atoms which undergoes pi-bond formation. And has pi orbitals of oxygen and carbon involved in the bonding process.
Answer 3) Bond b: O
 H has only S orbital involved in bonding.
H has only S orbital involved in bonding.
Explanation : The bonding at 'b' position involves oxygen
 hydrogen atoms in it. It has
hydrogen atoms in it. It has
 hybridized orbitals and S orbital of hydrogen involved in the bonding.
hybridized orbitals and S orbital of hydrogen involved in the bonding.
Answer 4) Bond c: C is
 O is also
O is also

Explanation : The bonding involved at 'c' position has carbon and oxygen atoms involved in it. Both the atoms involves the orbitals of
 hybridized bonds.
hybridized bonds.
Answer 5) Bond d: C atom has
 C atom has
C atom has

Explanation : At the position of 'd' the bonding between two carbon atoms is found to be
 . Therefore, the orbitals that undergo
. Therefore, the orbitals that undergo
 hybridization are
hybridization are
 .
.
Answer 6) Bond e : C1 containing O
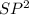
C2 is

Explanation : The carbon atom which contains oxygen along with a double bond has
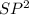 hybridized orbitals involved in the bonding process; whereas the carbon at C2 has
hybridized orbitals involved in the bonding process; whereas the carbon at C2 has
 hybridized orbitals involved during the bonding. This is for the 'e' position.
hybridized orbitals involved during the bonding. This is for the 'e' position.