The chemical reaction that occurs between
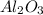 ,
,
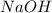 and
and
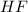 to form cryolite (molecular formula:
to form cryolite (molecular formula:
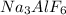 ) is:
) is:
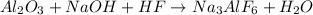
The balanced reaction is:
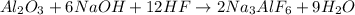
In order to determine the weight of cryolite produced, the limiting reactant (the reactant that is completely used up in the reaction) should be identified first. To determine the limiting reactant, the number of moles of each reactant with respect to cryolite (
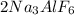 ) should be calculated.
) should be calculated.
The formula for determining the number of moles is:
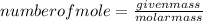
1. For
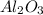 :
:
According to the balanced equation, 1 mole of
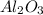 reacts to give 2 moles of
reacts to give 2 moles of
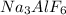 .
.
Molar mass of
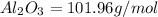
So,
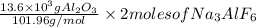
Number of moles =
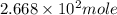
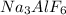
2. For
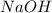 :
:
According to the balanced equation, 6 mole of
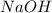 reacts to give 2 moles of
reacts to give 2 moles of
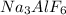 .
.
Molar mass of
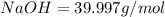
So,
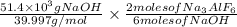
The number of moles =
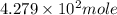
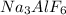
3. For
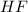 :
:
According to the balanced equation, 12 moles of
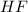 reacts to give 2 moles of
reacts to give 2 moles of
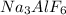 .
.
Molar mass of
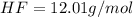
So,
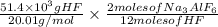
Number of moles =
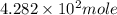
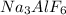 .
.
Thus,
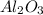 is the limiting reactant.
is the limiting reactant.
Molar mass of
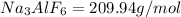
Amount of
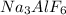 produced =
produced =
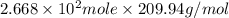 =
=
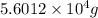
Since,
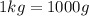
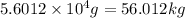
Hence, the amount of cryolite produced is
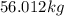 .
.