For simple cubic unit cell:
Let the edge length = a
Such that a = 2r (where r is the radius of the atom)
Then radius of the atom =
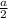
Volume of the unit cell =
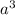
Number of atoms in simple cubic unit cell = 1.
Therefore, empty space = volume of cube - volume occupied by atom
Percentage of empty space =
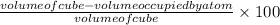
Substituting the values:
Percentage of empty space =
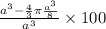
Percentage of empty space =

Percentage of empty space =
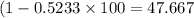 %.
%.
Hence,
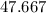 % is the empty space of the simple cubic unit cell.
% is the empty space of the simple cubic unit cell.