Dipole moment measures the polarity of the molecule that is measure of the separation of two opposite charges. Electronegativity difference between the atoms results dipole moment.
Dipole moment of bond is equal to the product of the magnitude of charge and distance between them (charges).
It is given by:
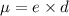
where,
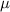 = dipole moment
= dipole moment
 = magnitude of charge whether partially positive or negative charge.
= magnitude of charge whether partially positive or negative charge.
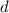 = distance between charges.
= distance between charges.
Since,
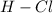 bond distance is larger than the
bond distance is larger than the
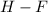 bond distance, because of large partial charge on the atoms of
bond distance, because of large partial charge on the atoms of
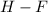 ; from the definition of dipole moment
; from the definition of dipole moment
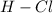 (1.08) has smaller dipole moment than
(1.08) has smaller dipole moment than
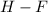 (1.82).
(1.82).