Answer:-

Explanations:- When the covalent bond is formed between two different atoms then the more electron negative atom has
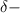 and the less electron negative atom has
and the less electron negative atom has
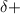 charge. More is the electron negativity difference, greater is the charge separation.
charge. More is the electron negativity difference, greater is the charge separation.
Looking at all the given molecules, Oxygen is most electron negative and hydrogen is less electron negative(hydrogen is more electron negative than Si). So, the best choice is
 .
.