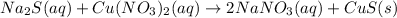In a chemical reaction one or more reactants combine or react to form one or more product(s) with different chemical and physical properties.
When aqueous sodium sulfide reacts with aqueous copper(II) nitrate results in the formation of aqueous sodium nitrate and solid copper(ll) sulfide.
First, identify the chemical symbols for the elements in the compounds.
Sodium sulphide =
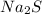
Copper(II) nitrate =
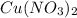
Sodium nitrate =
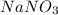
Copper(II) sulfide =
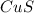
The chemical reaction is given as:
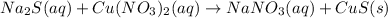
Now, balance the chemical equation:
For balance the equation, multiply
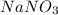 by 2.
by 2.
Thus,