The chemical formula for ammonium carbonate is (NH₄)₂CO₃ .
It shows that one mole of ammonium carboonate contains two mole of ammonium ion that is NH₄⁺ .
Number of moles of ammonium carbonate , n=
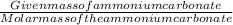
Given mass of the ammonium carbonate=0.557 g
Molar mass of the ammonium carbonate= 96.086 g/mol
Number of moles of ammonium carbonate,
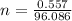
=0.0057 mol (NH₄)₂CO₃
0.0057 mol (NH₄)₂CO₃=0.0057×2 of NH₄⁺
=0.0114 of NH₄⁺