Answer:-
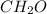 .
.
Solution:- From given grams of carbon dioxide and water we could calculate their moles. using mole ratio, moles of C and H are calculated. These moles of C and H are converted to grams and then we subtract the sum of grams of C and H from given mass of compound to calculate the grams of oxygen. Grams of oxygen are converted to moles and then we find out the mole ratio of C, H and O that gives us empirical formula.
The calculations are as follows:
moles of carbon dioxide =
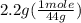 = 0.05 mole
= 0.05 mole
moles of water =
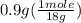 = 0.05 mole
= 0.05 mole
 has one C means the mole ratio of
has one C means the mole ratio of
 to C is 1:1. So, moles of C would also be 0.05.
to C is 1:1. So, moles of C would also be 0.05.
 has two H means the mole ratio of
has two H means the mole ratio of
 to H is 1:2. So, moles of H would be 2 times the moles of
to H is 1:2. So, moles of H would be 2 times the moles of
 which is 0.10.
which is 0.10.
Let's convert moles of C and H to their grams as:
grams of C =
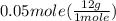 = 0.6 g
= 0.6 g
grams of H =
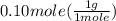 = 0.1g
= 0.1g
grams of O = 1.5 - (0.6 + 0.1)
grams of O = 1.5 - 0.7 = 0.9 g
moles of O =
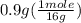 = 0.056 moles
= 0.056 moles
Let's calculate the mole ratio now:
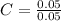 = 1
= 1
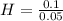 = 2
= 2
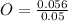 = 1
= 1
From the mole ratio, the empirical formula of fructose is
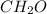 .
.