The volume of cylinder is 100 mL, inner diameter is 3.2 cm thus, radius will be:
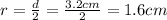
Volume of layer formed=
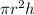 ...... (1)
...... (1)
Volume of layer is also equal to sum of volume of gasoline and water.
Density of gasoline is
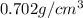 and mass is 34 g thus, volume of gasoline will be:
and mass is 34 g thus, volume of gasoline will be:
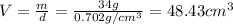
Now, density of water is
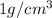 and mass is 34 g thus, volume of water will be:
and mass is 34 g thus, volume of water will be:
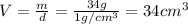
Adding both the volumes, volume of layer will be:
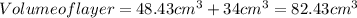
Putting the values in equation (1) to solve for height of the layer,
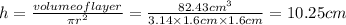
Thus, height of layer is 10.25 cm