So we know that this amount of atoms is Avogadro's number - the number of atoms in 1 mole of a substance.
So then, using dimensional analysis, we can find the mass, in grams, of potassium.
First we must find the molar weight of potassium as found on the periodic table: 39.098g/mol
Then we can solve directly for mass:
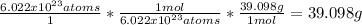
Therefore, the amount of atoms you have given, in terms of mass, will equal 39.098g - if the answer requires significant figures, then the mass would be 39.1g of potassium.