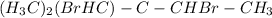Answer : 2,2-dimethylbutane actually has got 3 methyl radicals and 1 ethyl radical attached to the 2nd carbon.
The pairs of positions for the Br atoms are in these ways
(a) both on the far end of the ethyl
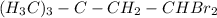
(b) both on the near C of the ethyl
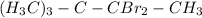
(c) both on the ethyl but not on the same C
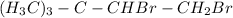
(d) both on one of the methyls

(e) both on methyls, but not the same methyl

(f) one on a methyl and one on far end of ethyl
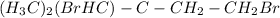
(g) one on a methyl and one on near C of ethyl.