Answer: D.
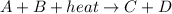
Explanation: An endothermic reaction is a type of of chemical reaction in which energy is absorbed from the surrounding. The temperature of the surrounding decreases.
An Exothermic reaction is a type of of chemical reaction in which energy is released into the surrounding. The temperature of the surrounding increases.
The chemical equation for an endothermic reaction is:
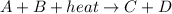 because the energy in the form of light has been absorbed by the reactants. The difference in the energies of reactants and products is the energy which was being absorbed on completion of reaction.
because the energy in the form of light has been absorbed by the reactants. The difference in the energies of reactants and products is the energy which was being absorbed on completion of reaction.