Answer:
The balanced chemical reaction is given as:
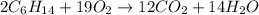
Step-by-step explanation:
Combustion is a type of chemical reaction in which hydrocarbon reacts with oxygen gas to form carbon dioxide gas and water .
When 2,3-dimethylbutane undergoes combustion reaction it gives carbon dioxde and water.
The balanced chemical reaction is given as:
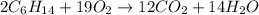
According to stoichiometry, 2 moles of 2,3-dimethylbutane reacts with 19 moles of oxygen gas to give 12 moles of carbon dioxide and 14 moles of water.