Chemical reaction is given as :
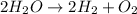
Here, 2 moles of water gives 2 moles of hydrogen and one mole of oxygen.
Mass of hydrogen = 28.0 g (given)
Mass of oxygen = 224.0 g (given)
Number of moles =
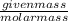
Thus, number of moles of hydrogen =
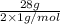
= 14 moles of hydrogen
Number of moles of oxygen=
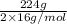
= 7 moles of oxygen
Now, to find the amount of water:
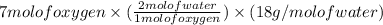
= 252 g of water.
Thus, amount of water is 252 g (involved in the process).