Answer:- Specific heat of glass is
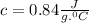 .
.
Solution:- It asks to calculate the specific heat of glass when 13.4 J of heat is required to heat 8.0 g of water by 2.0 degree C.
The equation used for solving this type of problems is:
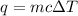
where, q is the heat energy, m is the mass, c is specific heat and
 is change in temperature.
is change in temperature.
From given info:
q = 13.4 J
m = 8.0 g
 = 2.0 degree C
= 2.0 degree C
c = ?
Let's plug in the values in the equation and solve it for c:
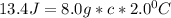
On rearrangement:
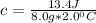
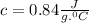
So, the specific heat of glass is
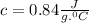 .
.