Answer:- Molarity of the acid solution is 0.045M.
Solution:- The balanced equation for the reaction of given acid and base is:
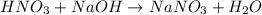
From the balanced equation, they react in 1:1 mol ratio. So, we could easily solve the problem using the equation:
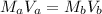
where,
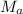 is the molarity of acid,
is the molarity of acid,
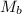 is the molarity of base,
is the molarity of base,
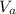 is the volume of acid and
is the volume of acid and
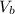 is the volume of base.
is the volume of base.
Let's plug in the given values in the equation:
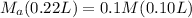
on rearranging the above equation:
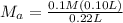
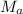 = 0.045M
= 0.045M
So, the molarity of the acid solution is 0.045M.