Answer:- Empirical formula is MgO.
Solution:- From given information,
Mg = 60.3%
O = 39.7%
Percentages could be considered as their grams and to convert them to moles we divide by their atomic masses.
moles of Mg =
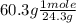 = 2.48 mole
= 2.48 mole
moles of O =
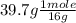 = 2.48 moles
= 2.48 moles
We have equal moles of Mg and O, it means the mole ratio is 1:1 and so the empirical formula is MgO.