Given, the density of a particular brand of gasoline = 0.737 g/ml.
13.4 gallon of tank given. We have to find the mass of gasoline to fill the tank.
First we will convert 13.4 gallon to ml.
We know that 1 gallon = 3.78541 litre
And 1 litre = 1000 ml
So we can write 1 gallon =
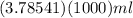 = 3785.41 ml.
= 3785.41 ml.
13.4 gallon =
 ml = 50724.494 ml.
ml = 50724.494 ml.
Let's take there is x grams of gasoline
We know that density = (mass/ volume) =
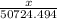
So we can write,
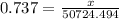
To find x we will multiply both sides by 50724.494. we will get,
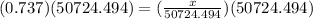
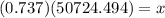
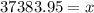
So we have got the required answer.
Mass of gasoline = 37383.95 grams.