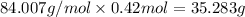The balanced equation between the
 and
and
 is:
is:
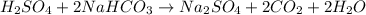
Formula of molarity is:
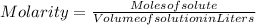
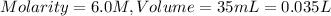
Substituting the values,
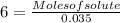
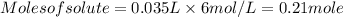
So, number of moles of
 is
is
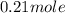 .
.
From the balanced equation it is clear that for 1 mole of
 , 2 moles of
, 2 moles of
 are required.
are required.
Hence, 0.21 mole of
 =
=
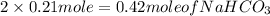
Molar mass of
 =
=
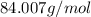
So, the mass of
 =
=