Answer: The pH of the solution is 0.78
Step-by-step explanation:
To calculate the molarity of the solution after mixing 2 solutions, we use the equation:
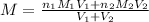
where,
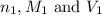 are the n-factor, molarity and volume of the HCl
are the n-factor, molarity and volume of the HCl
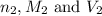 are the n-factor, molarity and volume of the HCl
are the n-factor, molarity and volume of the HCl
We are given:
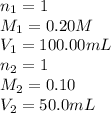
Putting all the values in above equation, we get:
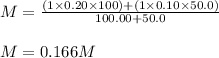
The concentration of
 ions in the resulting solution will be same as the molarity of solution which is 0.166 M.
ions in the resulting solution will be same as the molarity of solution which is 0.166 M.
pH is defined as negative logarithm of hydrogen ion concentration. It is basically defined as the power of hydrogen ions in a solution.
Mathematically,
![pH=-\log[H^+]](https://img.qammunity.org/2019/formulas/chemistry/high-school/vwilut25e4cux34589pwoorivy6w6y51xe.png)
We are given:
![[H^+]=0.166M](https://img.qammunity.org/2019/formulas/chemistry/high-school/zdfn0r4ft3ylsufdeolqmg48zbk8e6xgvr.png)
Putting values in above equation, we get:
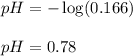
Hence, the pH of the solution is 0.78