Answer:
9.6936 grams of sodium bicarbonate are needed to make 23.75ml of a 4.861 M solution.
Step-by-step explanation:
Sodium bicarbonate is a compound of formula NaHCO₃.
Molarity is defined as the number of moles of solute (substance in minor proportion in a solution) in a given volume.
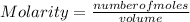
Molarity is expressed in units (moles / liter).
So, 4,861 M means that in 1 L of solution there are 4,861 moles. Knowing that 23.75 mL equals 0.02375 L (1 L = 1000 mL), it is possible to apply a rule of three as follows: if in 1 liter of solution there are 4,861 moles of sodium bicarbonate, in 0.02375 L of solution, how many moles of the compound there is?
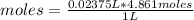
moles=0.1154
On the other side, the atomic mass of the elements that make up the sodium bicarbonate are:
- Na: 23 g/mol
- H: 1 g/mol
- C: 12 g/mol
- O: 16 g/mol
So, the mass of the sodium bicarbonate is:
NaHCO₃: 23 g/mol + 1 g/mol + 12 g/mol + 3*16 g/mol= 84 g/mol
Then, a rule of three can be applied to know the mass of sodium bicarbonate that are needed to make 23.75ml of a 4.861 M solution. The rule of three applies as follows: if, according to the molar mass of the sodium bicarbonate, in 1 mole there are 84 grams, in 0.1154 moles (previously calculated moles) how many grams are present?
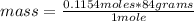
mass=9.6936 grams
9.6936 grams of sodium bicarbonate are needed to make 23.75ml of a 4.861 M solution.