The formula of determining the
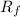 value is:
value is:
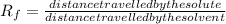
where both the distance are measured from the common origin.
10 % ethyl acetate/ hexane means it has 10 % ethyl acetate and 90 % hexane which are non-polar in nature. Hence, the non-polar compound will travel farther in the TLC plate along with the eluent and the polar compound will travel less as they remain attracted to the polar adsorbent
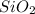 .
.
Among 3-decanone and 3-decanol, 3-decanone will have high
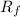 as it is non-polar.
as it is non-polar.
Among toluene and benzoic acid, toluene will have high
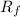 as it is non-polar.
as it is non-polar.
Among cyclooctane and cyclooctanone, cyclooctane will have high
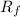 as it is non-polar.
as it is non-polar.