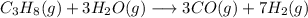Answer:
Step-by-step explanation:
Hi, as it says you have two reactants: propane gas (
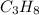 ) and steam (
) and steam (
 ). This reaction have two products: carbon monoxide (
). This reaction have two products: carbon monoxide (
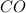 ) and hydrogen gas (
) and hydrogen gas (
 ).
).
All compounds in the reaction are in gas state due to the high Temperature.
The reaction equation:
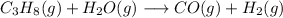
Balancing: