Answer : The correct option is, (B)
![([H_2O])/([H_2S])](https://img.qammunity.org/2019/formulas/chemistry/high-school/vcem14vl7bk0nd6vz2r0kj1xu3b9rkav83.png)
Explanation :
The given balanced chemical reaction is,
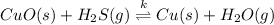
This reaction is a reversible reaction.
The rate of forward reaction will be,
![Rate=k_f[H_2S]](https://img.qammunity.org/2019/formulas/chemistry/high-school/t75mu7agwygg9br96inr4lwu7dsxnuqxx6.png)
The rate of backward reaction will be,
![Rate=k_b[H_2O]](https://img.qammunity.org/2019/formulas/chemistry/high-school/s6pycmay785zqofqhbd0qtmhzh31sck3f2.png)
And at equilibrium the rate of reaction is equal to the rate of backward reaction divided by the rate of forward reaction.
![(k_b)/(k_f)=([H_2O])/([H_2S])](https://img.qammunity.org/2019/formulas/chemistry/high-school/t489e3tkrdvd0a71hwr688lfrysiz42joz.png)
or,
![K=([H_2O])/([H_2S])](https://img.qammunity.org/2019/formulas/chemistry/high-school/9193g3c74i7ww67wa2ynl3v67tm39enh61.png)
Hence, the correct expression for the equilibrium constant is,
![K=([H_2O])/([H_2S])](https://img.qammunity.org/2019/formulas/chemistry/high-school/9193g3c74i7ww67wa2ynl3v67tm39enh61.png)