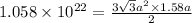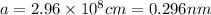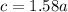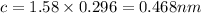(a)
The volume of unit cell is given by:
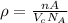
where
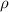 is density, n is the number of atoms in the given HCP crystal, A is the atomic weight of titanium,
is density, n is the number of atoms in the given HCP crystal, A is the atomic weight of titanium,
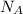 is the Avogadro's number.
is the Avogadro's number.
Putting the values in the formula:
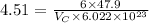
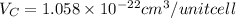
Thus, the volume of unit cell is
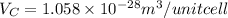
(b)
The edge length of HCP is given by:
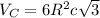
where R is the atomic radius of the atom and c is the height of hexagon.
Substituting
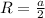 in the above equation of
in the above equation of
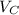 :
:
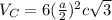 =
=
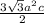
Substituting the values: