Answer : This is homogeneous catalysis. Br⁻ is behaving as a catalyst
Explanation :
Let us write down the given reactions.
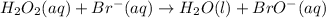 .....Slow
.....Slow
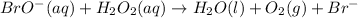 .....Fast
.....Fast
A catalyst is defined as a substance that increases the rate of the reaction.
A catalyst takes part in the reaction by reacting with the reactants to form an intermediate. But it is regenerated at the end of the reaction.
If we take a look at the above reactions, we can see that bromide ion Br⁻ is reacting with H₂O₂ to form an intermediate BrO⁻. It is also getting regenerated back at the end. That means Br⁻ is behaving as a catalyst.
We can also see that the reactant and the catalyst are in the same phase which is aqueous phase. Therefore the reaction is homogeneous.
A homogeneous reaction is the one where the reactants and the catalyst are in the same phase.