Answer:- Answer is 69.7 second.
Solution:- For solving problems based on first order equations is:
![ln[A]=-kt+ln[A_0]](https://img.qammunity.org/2019/formulas/chemistry/high-school/kwfaeg0dlaxc2ktwruk0v3sus0x8ow9z73.png)
where,
![[A_0]](https://img.qammunity.org/2019/formulas/chemistry/high-school/5iss4o0vwlydam3jwv0galzd0ve0u83dug.png) is the initial concentration and [A] is final concentration.
is the initial concentration and [A] is final concentration.
k is rate constant and t is the time.
It asks to calculate the time in which 30% of the substrate would be consumed. Let's say the initial amount of the substrate is 100 then 30 is used and remaining would be, 100 - 30 = 70
So,
![[A_0]](https://img.qammunity.org/2019/formulas/chemistry/high-school/5iss4o0vwlydam3jwv0galzd0ve0u83dug.png) = 100
= 100
[A] = 70

t = ?
Let's plug in the values in the equation and solve it for t:
![ln[70]=-5.12*10^-^3(t)+ln[100]](https://img.qammunity.org/2019/formulas/chemistry/high-school/1hp242308xnlmge4rht2l5g7rwjbuxb75b.png)
4.248 = -0.00512(t) + 4.605
4.248 - 4.605 = -0.00512(t)
-0.357 = -0.00512(t)
we have negative sign on both sides, so it is canceled out.
0.357 = 0.00512(t)
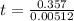
t = 69.7 seconds
So, it would take 69.7 seconds for 30% substrate to be consumed.