The formula of the density is given as:
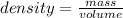
Rearranging the formula as:
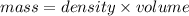
Substituting the values in the above formula:
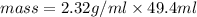
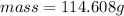
No, the result is not in the accordance with the rules for significant figures. As the mass of the liquid have 6 significant figures whereas if the rules of significant figures are applied then the mass must be in 3 significant figures.